
New Gains Seen from Patient- and Site-Centric Initiatives
This white paper is part of DIA’s White Paper Library
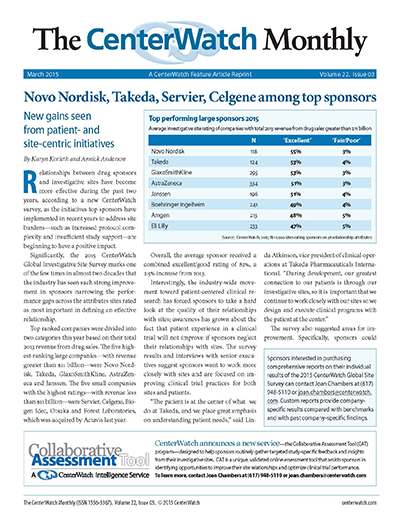
Relationships between drug sponsors and investigative sites have become more effective during the past two years, according to a new CenterWatch survey, as the initiatives top sponsors have implemented in recent years to address site burdens - such as increased protocol complexity and insufficient study support - are beginning to have a positive impact. Significantly, the 2015 CenterWatch Global Investigative Site Survey marks one of the few times in almost two decades that the industry has seen such strong improvement in sponsors narrowing the performance gaps across the attributes sites rate as most important in defining an effective relationship.
Download Now!
INTERESTED IN PUBLISHING?
For more information on publishing your White Paper with DIA, including schedule and pricing, please contact Heej Ko at [email protected].
For more information on publishing your White Paper with DIA, including schedule and pricing, please contact Heej Ko at [email protected].